Classical physics or Newtonian physics is capable of explaining and describing the events happening around us in a precise and accurate way. For example, if you kick a football it can perfectly explain how much force will be exerted the path it will follow and how far it will travel.

Newtonian physics is very precise and useful for analyzing and explaining the events and phenomena of our everyday lives. However it is impossible to explain events at the atomic and subatomic level using Newtonian physics. Towards the end of the 19th century and the beginning of the 20th century, physicists observed some strange phenomena at the atomic and sub-atomic level that could not be explained by the conventional laws of Newtonian physics. In other words Newtonian physics became completely ineffective at describing events at the sub-atomic scale. In an attempt to explain these strange phenomena, a new chapter in physics was born which is known as Quantum Physics.
Albert Einstein’s theory of relativity explains large-scale objects in the universe, whereas quantum physics is capable of precisely explaining the behavior of matter at the smallest scales. Quantum physics is a very complex subject, so it must be learned and understood step by step, and it is impossible to grasp it all at once. Therefore, we will try to explore quantum physics from the beginning, step by step, to the end (such as: Double Slit Experiment → Wave-Particle Duality → Photoelectric Effect → Quantum Entanglement → Quantum Tunneling → Quantum Field Theory → Quantum Teleportation → Schrödinger’s Cat → Quantum Fluctuation → Quantum Gravity → Quantum Electrodynamics → Feynman Diagrams, etc.).
In the introductory phase of quantum physics we will discuss some of the most important topics such as the Double Slit Experiment Wave Particle Duality and the Photoelectric Effect.
The double slit experiment was the first to show that light has both wave like and particle like properties meaning light can behave as both a wave and a particle.
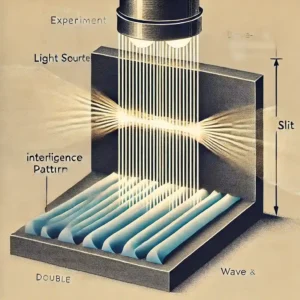
In 1801 Thomas Young conducted this double slit experiment using light demonstrating its wave like nature. At that time it was believed that light was composed solely of particles and had no wave like properties. The results of Thomas Young’s experiment contradicted that belief.
In 1909, British physicist G.I. Taylor repeated Thomas Young’s double-slit experiment, but instead of direct light, he used single photons.
In this experiment, Taylor observed single spots on the screen when single photons were used. If light were purely wave-like, there would be no single spots for a single photon.
Taylor repeated the experiment but this time used multiple photons and when he did he observed an interference pattern on the screen. This result led to the understanding that individually a photon behaves like a particle but collectively multiple photons behave like a wave.
he intrigue of wave-particle duality extended further when physicists performed the double-slit experiment using electrons instead of photons The scientists were shocked because electrons which were long thought to be purely particles were now behaving like waves. What an amazing discovery!
Furthermore an even stranger phenomenon was observed. During the double slit experiment if a detector was used to observe or measure the electrons they no longer behaved like waves but instead exhibited particle like properties.
When no observation or measurement was made the electrons reverted to their wave like behavior. This strange behavior of electrons gave rise to a deep mystery. When electrons are being observed they behave like particles but when they are not observed they act like waves.
You might ask what does it mean for electrons to behave like waves?
An easy example of a wave is the ocean’s waves. We’ve all seen ocean waves, either in real life or on television. Just like how ocean waves rise and fall, waves also have a similar up-and-down motion.
Later, Erwin Schrödinger provided an equation to describe the shape of the electron’s wave, but his explanation was not very comprehensible. In 1926 German physicist Max Born provided an excellent interpretation of the wave equation. Born stated that the wave equation is related to probability.
Since the electrons appear on the screen as waves it is difficult to pinpoint exactly where the electron will appear as a particle on the screen.
According to Born’s interpretation where the wave is larger there is a higher probability of finding the electron and where the wave is smaller the probability is lower. This means that no one can definitively say where the electron is located.
We can only give the probability of where the electron might be found. Even though it sounds strange, this is how the wave-like properties of electrons are explained.
When you fire an electron through a double slit, you cannot predict where the electron will hit the screen! However using Schrodinger’s wave equation you can calculate the probability of the electron hitting a specific spot on the screen.
Schrodinger’s wave equation and Max Born’s interpretation tell us how the electron will behave in its orbit and what the shape of its orbit will be.
In an atom we can only give the probability of where an electron will be orbiting around the nucleus but we cannot say with certainty. Because we are talking about the probability of the electron being in all possible locations at the same time.
When we try to observe or measure where the electron is we find it at a specific location. When we are not trying to observe or measure the electron it exists as a wave everywhere at once.
But why can’t we predict exactly where the electron is? The most widely accepted explanation for this was given by Werner Heisenberg and Niels Bohr. Their explanation is known as the Copenhagen Interpretation.
According to this interpretation until we try to observe where the electron is it exists everywhere at once and in all places simultaneously. The strange behavior of the electron might make you think it has some sort of consciousness or mind of its own.
According to Niels Bohr, when an electron or any sub-atomic particle is observed, the measurement force collapses the electron’s possible states into one specific location. As a result we only see the particle at one particular place.
In the double-slit experiment, a light source such as a laser is used to illuminate a sheet, allowing light to pass through two parallel slits. The light passing through the slits is observed on a screen placed behind the sheet. By looking at the images below you can better understand this concept.
When light passes through the two slits it creates an interference pattern on the screen with bright and dark bands (similar to the way different colors of the rainbow form a band together). This proves that light has wave like properties.
Even though the light passing through the slits always appears to be absorbed on certain parts of the screen, it does so in a way that is characteristic of a particle rather than a wave.
What a strange thing! The interference pattern is caused by the density of the particles hitting the screen, but in other experiments (where detectors were used in the slits), the particles behaved like particles. These experiments demonstrated the principle of wave-particle duality.
According to our previous belief if light were purely made of particles it should create a pattern on the screen that mirrors the shape and size of the slit when it passes through a single slit.
However when the experiment was conducted with a single slit, the diffraction pattern on the screen showed that light was spreading out. This means the smaller the slit, the larger the angle of the spread.
When a red laser is used to illuminate a single slit, the pattern created on the screen has a central bright spot, and if observed closely, two faint light bands can also be seen above and below the central spot.
With better instruments even more light bands can be observed. This diffraction pattern is the result of light’s wave like properties passing through the slit.
If two parallel slits are illuminated the light passing through them will interfere with each other producing a much clearer interference pattern of alternating bright and dark bands on the screen.
The width of these bands is a characteristic of the wavelength of the light.
When Thomas Young first demonstrated this process in the early 1800s, it became clear that light is made of waves. Young’s experiment disproved Isaac Newton’s theory (proposed in the 17th and 18th centuries) that light was made of particles and played a key role in the acceptance of the wave theory of light.
Interestingly later discoveries the photoelectric effect showed that in certain situations light behaves as if it is made of individual particles.
This contradiction between wave like and particle like behavior is a core feature of quantum physics and it led to the development of the theory of the quantum nature of light.
Wave particle duality refers to the phenomenon where electromagnetic waves (like light) exhibit both wave like and particle like behaviors simultaneously.
This is because the behavior of electromagnetic waves cannot be fully explained using only wave or particle properties alone. This led to the development of the wave particle duality theory.
To explain properties such as diffraction, convergence, reflection, scattering, refraction and interference of electromagnetic waves one must use the wave nature of electromagnetic radiation.
On the other hand phenomena like the photoelectric effect blackbody radiation and color changes due to heat are explained using the particle nature of electromagnetic waves. The wave particle duality theory is a fundamental aspect of quantum physics.
The Greek philosopher Democritus argued that light is composed of numerous tiny particles.
In the early 11th century the Arab scientist Ibn al-Haytham suggested that light rays are made of light particles.
In 1630 Rene Descartes described light’s behavior as waves in a universal medium like the waves of the ether.
Afterward in the 1670s Sir Isaac Newton developed his corpuscular theory providing a rational basis for it.
Newton argued that the straight lines of light reflection exhibit the particle nature of light and that light travels only along these straight lines. According to Newton’s reasoning light is composed entirely of particles. However in 1801 Thomas Young’s double slit experiment demonstrated light’s wave nature through the phenomenon of interference.
In 1803 Scottish physicist James Clerk Maxwell discovered that spontaneous waves generated by electric and magnetic fields could be described using his Maxwell equations with slight modifications. Maxwell easily proved that visible light, ultraviolet rays, infrared rays, and all other visible and invisible light rays are electromagnetic waves, possessing both particle and wave properties.
In 1900, German physicist Max Planck found that treating electromagnetic radiation as composed of specific particles allowed for the formation of an accurate mathematical theory of thermal radiation.
Building on Planck’s idea, in 1905, Albert Einstein resolved the mysteries of the photoelectric effect, which could not be explained by the wave theory of light. This discovery reinforced both the wave and particle nature of light.
In 1926, Gilbert Lewis coined the term “photon” for light particles. British physicist Paul Dirac was the first to propose a complete quantum theory of electromagnetic radiation.
Over time, Einstein’s theory of special relativity and modern quantum field theory advanced and expanded the understanding of quantum optics. According to the photoelectric effect, light consists of photons emitted in packets.
Numerous small particles combine to form waves, meaning multiple photons behave like waves together and like particles individually.
A photon is a quantum particle that behaves like an energy packet. The amount of energy in a wave packet is proportional to the wave’s frequency as expressed by the equation E = hf
where:
E = Energy,
h = Planck’s constant,
f = frequency of the light.
The photoelectric effect is a crucial concept in quantum physics. It explains how electrons are emitted from a material when it absorbs energy from incident electromagnetic waves (light).
Electrons emitted from metallic surfaces are known as photoelectrons. Heinrich Hertz discovered the photoelectric effect which led to the concept of wave particle duality.
When electromagnetic radiation falls on a metal plate the photons in the radiation are absorbed causing electrons to be emitted generating electric current. For example if low-wavelength high-frequency light (like gamma rays, X-rays, ultraviolet rays) is shone on a metal plate electrons will be emitted.
However if long-wavelength low-frequency light (like visible light, microwaves, radio waves) is used electrons won’t be emitted because the frequency of the incident radiation must be equal to or greater than the threshold frequency for electron emission.
If the frequency is lower the electrons in the metal plate won’t have enough energy to overcome the binding force and no electrons will be emitted.
In 1905 Albert Einstein’s equation of the photoelectric effect demonstrated that increasing the frequency of the incident radiation increased the energy of the emitted photoelectrons.
It wasn’t experimentally confirmed until 1915 when physicist Robert Andrews Millikan found that increasing the frequency of incident radiation linearly increased the energy of the emitted electrons proving Einstein’s equation to be correct.
Einstein was awarded the Nobel Prize in 1921 for his contribution to understanding the photoelectric effect.
The photoelectric effect can be demonstrated experimentally. In this experiment two zinc plates are placed in an evacuated quartz tube. One plate is coated with a thin layer of an alkali metal (lithium dioxide) and the other is left uncoated.
The coated plate is connected to the negative terminal of a battery while the uncoated plate is connected to the positive terminal via a galvanometer.
When electromagnetic radiation falls on the coated plate electrons are emitted which travel towards the uncoated plate creating an electric current (since the flow of electrons constitutes electricity).
The strength of the current depends on the intensity of the incident radiation’s frequency. If the frequency increases more electrons are emitted and the current increases. If the frequency decreases the current decreases as well.
In 1905, Albert Einstein explained this process through his photoelectric equation. When light radiation hits a metal plate, the electrons are struck by photons. As a result, the electrons absorb the energy of the photons (since photons are energy packets) and use it to escape from their bonds.
Once free, the electrons gain kinetic energy from the remaining photon energy, allowing them to scatter. The energy required to free the electron and the electron’s kinetic energy together equal the energy of the striking photon thus conserving energy.
Notably one photon can only free one electron because the entire energy of a photon is absorbed by a single electron. The electrons emitted from the metal plate are called photoelectrons.
This process can be expressed by Einstein’s photoelectric equation:
E(photon) = W(electron) + E(kinetic)
Where:
Understanding Photoelectric Effect in Quantum Physics
The photoelectric effect gave serious credence to the quantization of energy and the particle-like properties of light.
It showed that light can behave both as a wave and as a particle, depending on the context-a phenomenon known as wave-particle duality. This was one of the first pieces of evidence that led to quantum mechanics, revolutionizing our understanding of light and matter.
In summary, the photoelectric effect plays a pivotal role in our understanding of quantum physics, illustrating the interplay between light, energy, and matter. It shows how light, once thought of solely as a wave, can also act as a stream of energy packets, or photons, that interact with the particles of matter.
Email: info@universaldiary.com
© 2024 Universal Diary. All rights reserved